INYO COUNTY, December 22, 2020 – During the weekend, the much-awaited Pfizer-BioNTech vaccination distribution began at Northern Inyo Healthcare District with Emergency Department Registered Nurse Cameron Winston receiving the first dose. The historic moment may very well mark the moment of change in the outcome of the pandemic for Inyo County.
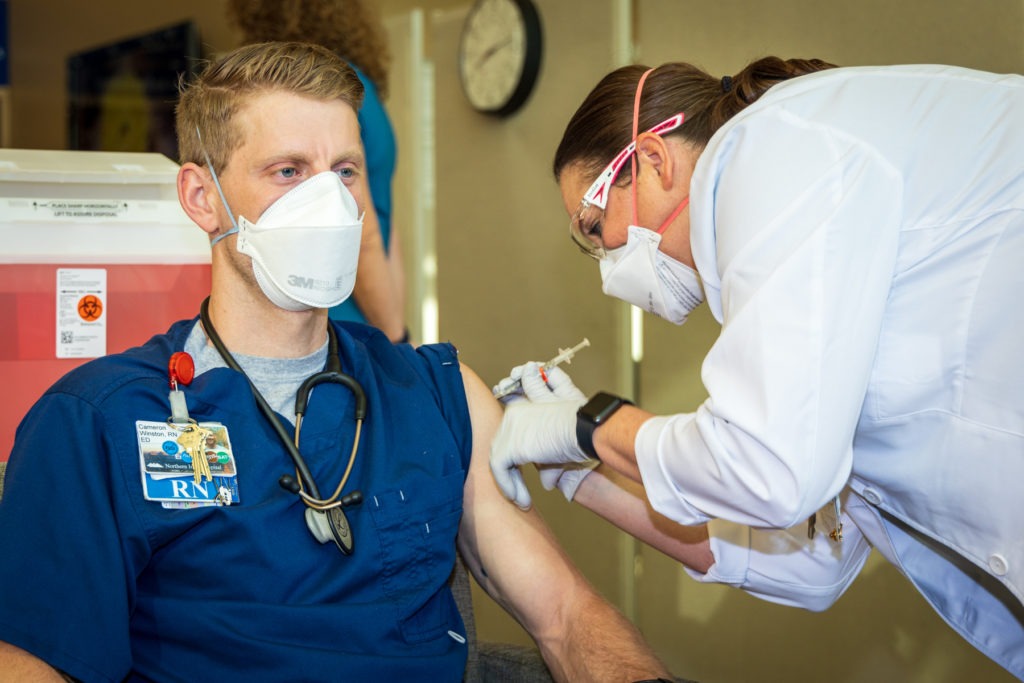
Cameron Winston, an Emergency Department Registered Nurse at Northern Inyo Hospital was the first person to receive the Pfizer-BioNTech COVID-19 vaccine at Northern Inyo Healthcare District. NIHD’s Chief Nursing Officer Allison Partridge administered Cameron’s vaccination on Saturday, Dec. 19, 2020. Cameron, along with other who received the vaccine, will be back for the required second dose on Jan. 9. (Photo by Barbara Laughon/Northern Inyo Healthcare District)
The County of Inyo and Northern Inyo Healthcare District stress that with last Thursday’s arrival of the Pfizer vaccine, all available vaccine will be offered to healthcare workers, including non-clinical personnel, and first responders within the 1A Tier of the California Department of Public Health (CDPH) Allocation Guidelines. It’s our hope that this allocation will be enough for everyone within the 1A tier. Should there be leftover doses, they will be offered to 1B individuals as defined and prioritized by the California Department of Public Health.
Under those guidelines, those eligible for the 1A tier vaccination include, as it is written, “persons at risk of exposure to SARS-CoV-2 through their work in any role in direct health care or long-term care settings. This population includes persons at direct risk of exposure in their non-clinical roles, such as, but not limited to, environmental services, patient transport, or interpretation.”
In addition, the first 100-dose shipment of the second FDA-approved vaccine from Moderna arrived at NIHD today, with subsequent shipments from Pfizer in two to four weeks. The initial Pfizer shipment was shared between Inyo and Mono counties, with Inyo receiving 615 doses and Mono receiving 360 doses.

















You are missing the point.
***To date there have been no mRNA vaccines licensed for use in humans.**
I’m not missing the point. I was pointing out your false statements, calling mRNA “new vaccine technology” and the Covid-19 vaccines to be “the first ever mRNA vaccines to actually be tested on humans.” both wholly inaccurate statements. I was just correcting you. You criticized Sierra Wave for “spouting misinformation”… Read more »
While you guys are playing Mr.Right ,I can’t find a person or #that can provide me with information on getting the vaccine ,She is a diasylis patient that meets the requirements by a long shot .3,patients out of her unit died last week. .a number ,a name ,a company ,anyone… Read more »
Didn’t an employee there experience a adverse allergic reaction of bubbling hives on her skin ??
“In addition, the first 100-dose shipment of the second FDA-approved vaccine from Moderna arrived at NIHD today, with subsequent shipments from Pfizer in two to four weeks.” Hey Eastern Sierra news staff, maybe you should do a little more research before you spout off misinformation. The Food and Drug Administration… Read more »
Our information is correct and based on information that we receive from our local healthcare districts, doctors, public health officers, and the CDC, as well as other reliable sources. Aside from your playing with semantics, we suggest that you take another run at the “facts,” and look for credible sources… Read more »
Hey Keith, “maybe you should do a little more research before you spout off misinformation.” mRNA is not new vaccine technology.
The Covid-19 vaccines are the first ever mRNA vaccines to be used in humans. You are correct that the concept of MRNA vaccines is not new. However these are the first ever MRNA vaccines to actually be tested on humans. No long term studies have ever been done with this… Read more »
There have been prior clinical trials using mRNA vaccines in humans, for rabies prevention. Here ya go: Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Alberer M, Gnad-Vogt U, Hong HS, Mehr KT, Backert L, Finak G, Gottardo… Read more »
Here’s another clinical trial started over a year ago with Moderna in humans…. “A Phase 1, Randomized, Observer-Blind, Placebo-Controlled, Dose-Ranging Study to Evaluate the Safety, Tolerability, and Immunogenicity of Zika Vaccine mRNA-1893 in Healthy Flavivirus Seropositive and Seronegative Adults” start date: July 30, 2019 In fact, there are at least… Read more »
Hey Keith – stop spreading misinformation and knock off the fear mongering. Firstly, COVID vaccines have been in development for 17 years and MRNA vaccines have been in development for 30 years. So yes, long term studies have been in place. Secondly, there is no data back yet on whether… Read more »